Submission of applications for human research projects involving persons
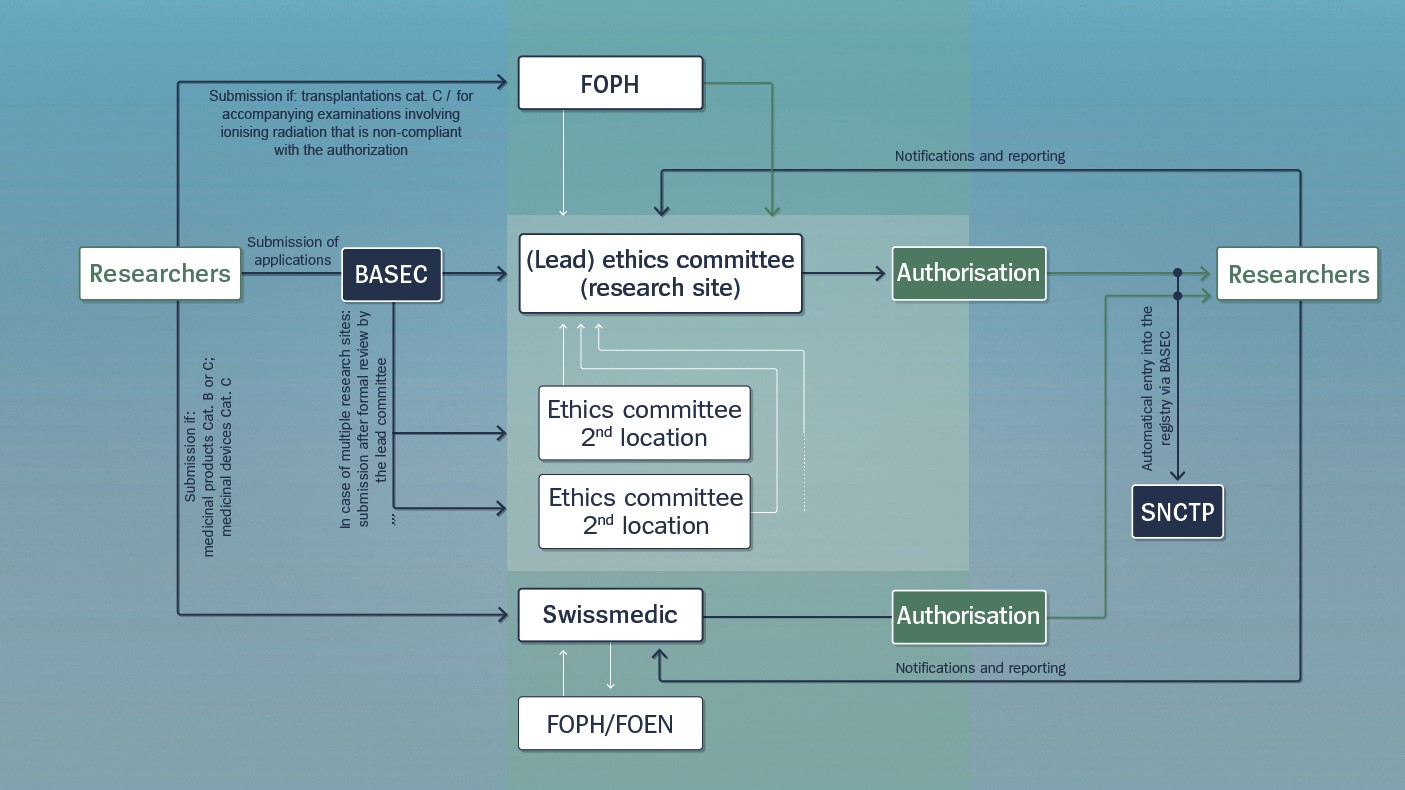
In Switzerland, submission of applications for human research projects involving persons takes the following course:
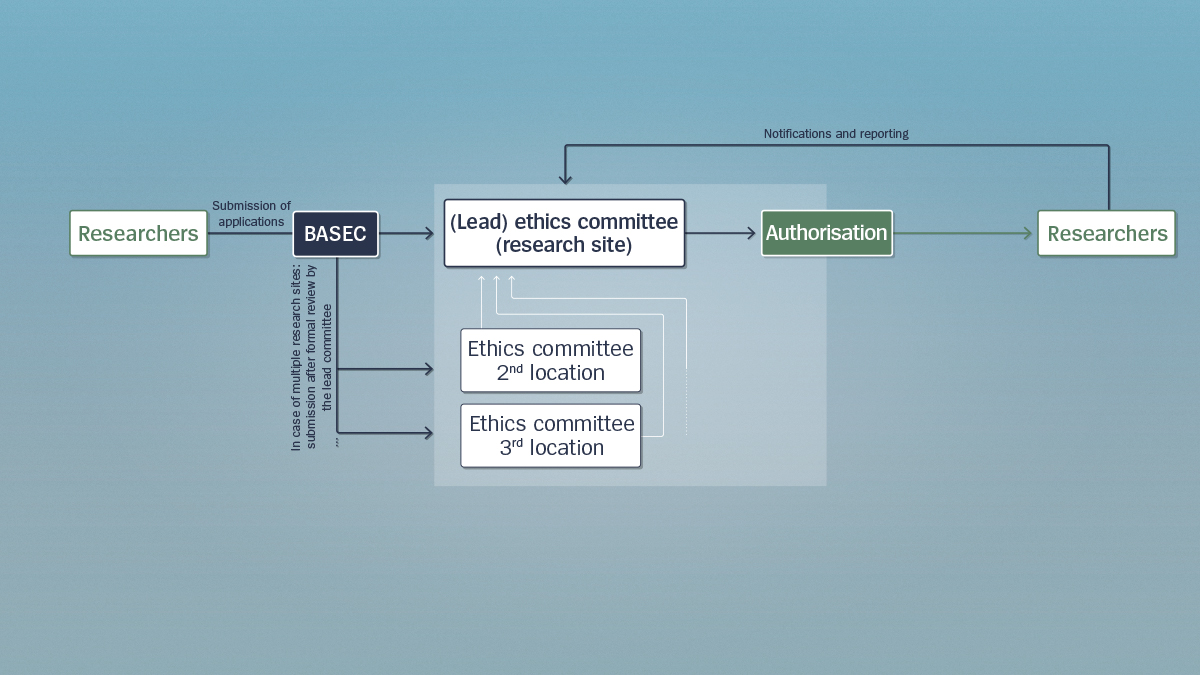
Researchers must submit their application via BASEC to their local ethics committee. The ethics committee reviews the application and grants the researchers authorisation to conduct their project, provided it fulfils the statutory requirements. During the study, researchers are obliged to report any unusual incidents to the ethics committee.
In the case of projects where research is being conducted in several locations in Switzerland, after initial formal review by the lead ethics committee, researchers must also submit their application to the other ethics committees affected.
In the case of clinical trials, once the application is approved, researchers must publish their project in the research register SNCTP.
In the case of projects that require authorisation by Swissmedic, researchers must also submit their application to Swissmedic. Depending on the situation, when reviewing the application, Swissmedic may consult with the FOPH or the FOEN. The duty for researchers to report unusual incidents also applies with regard to Swissmedic.
In the case of projects where transplants or investigations involving ionising radiation sources are performed, researchers must also submit their application to the FOPH.