Anonymised data sets
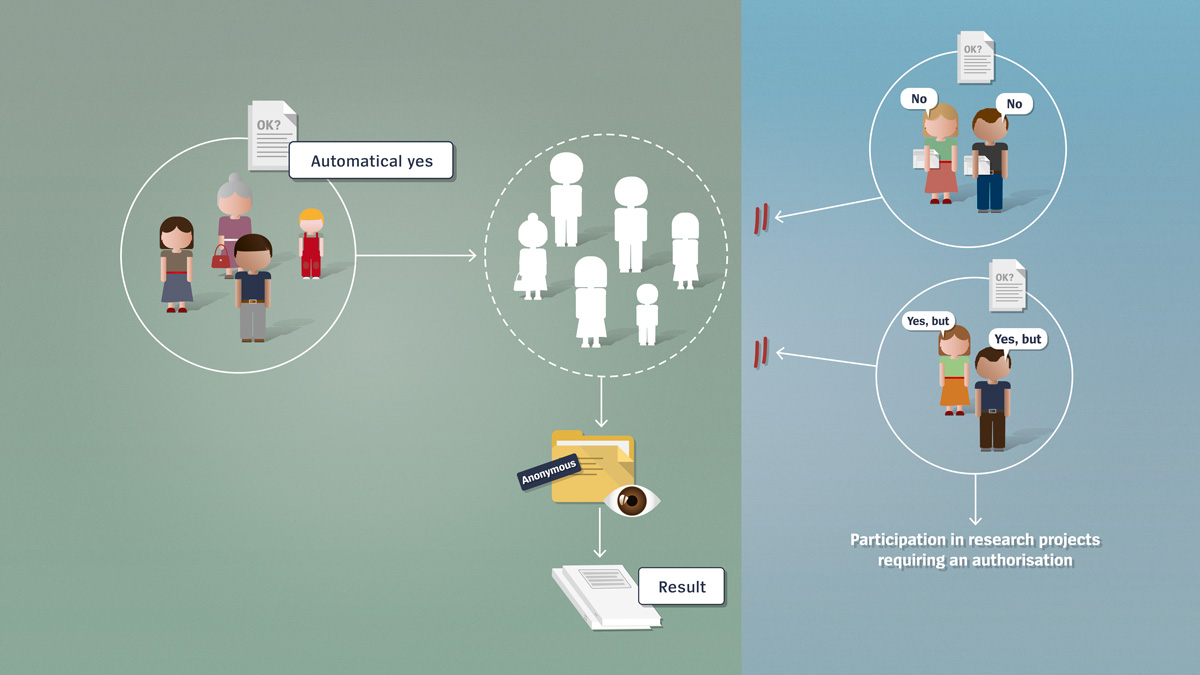
Projects that do not require authorisation always use anonymised data sets. In other words, the analysed data can no longer be assigned to a specific person.
Right to dissent with regard to anonymisation
Donors of biological material or genetic data are informed about the anonymisation in advance. If they do not file an objection, further use for research purposes can be made of their samples or data. All persons have the option of refusing to allow any further use of their samples or data. No prior notification is necessary in the case of studies with non-genetic data.
Foregoing anonymisation
Data donors can also deliberately forego anonymisation of their data in order to benefit from any possible study findings. In that case, however, they have to participate in a study that requires authorisation, as only these types of projects are permitted to work with non-anonymised data.