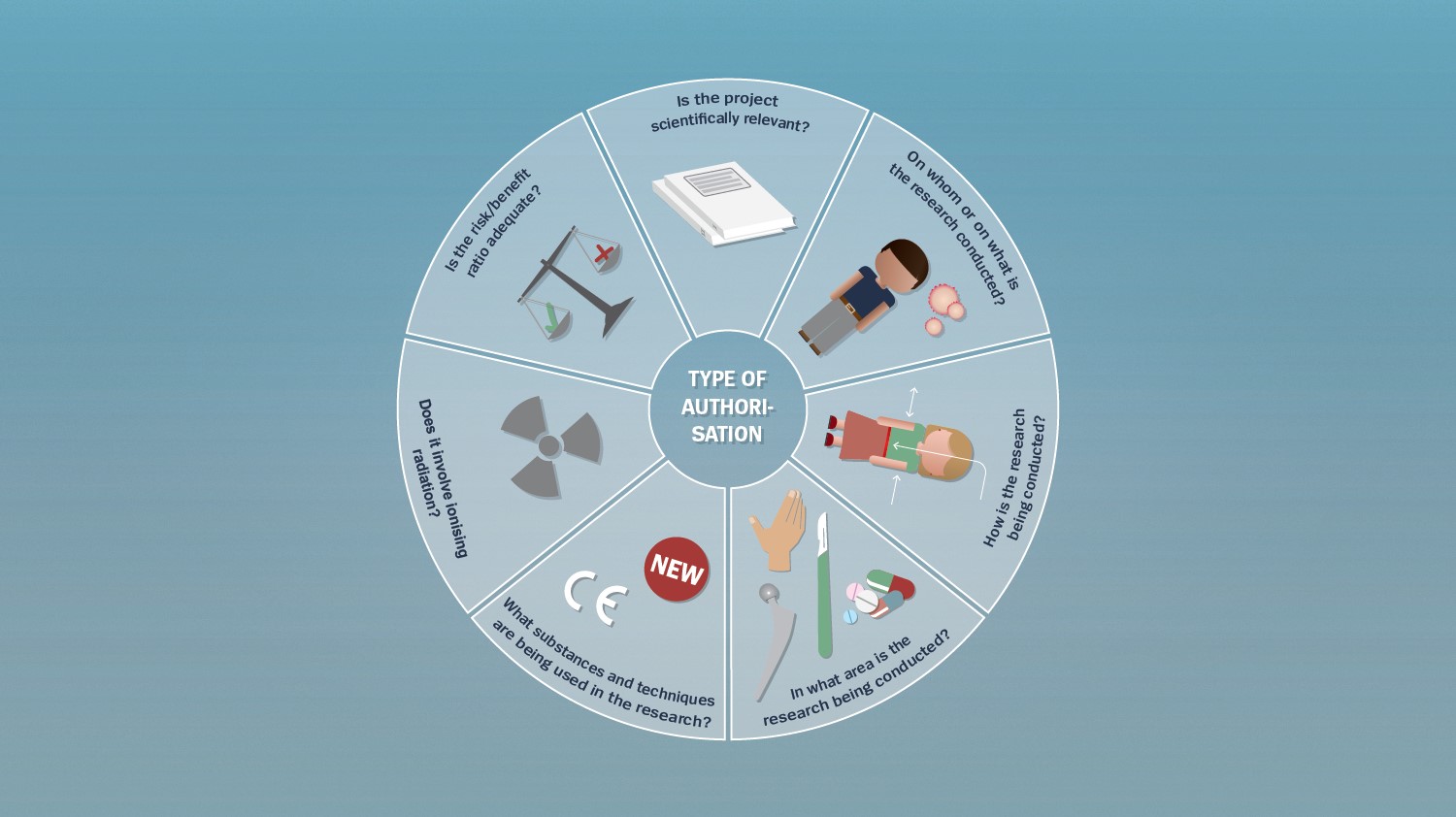
Various determinants
Human research projects are classified and reviewed according to the statutory requirements on the basis of various factors. Researchers as well as authorisation authorities ask themselves various questions related to the classification and review of a project:
Is the project scientifically relevant?
The first question establishes whether the research project actually aims at a scientifically relevant advancement of knowledge.
On whom or on what is the research conducted?
This question concerns the research subject: Is the research being conducted on living or deceased persons or on embryos or foetuses from abortions? Are health-related data or biological material being analysed?
How is the research being conducted?
This question concerns the type of research: Are participants being actively influenced or is the research purely observational? Are health-related personal data anonymised or encrypted?
In what area is the research being conducted?
The question here is about the types of procedures that are being implemented – these could be medicinal products, medical devices, therapies, other health-related interventions (such as an operation) or observational procedures.
What substances and techniques are being used in the research?
This question addresses the status the utilised substances or techniques have in Switzerland – are they approved and established, being used for purposes other than those intended or unauthorised?
Does it involve ionising radiation?
This question establishes whether the ionising radiation dose is within or above the statutory threshold value.
Is the risk/benefit ratio adequate?
Finally, the focus is on establishing the relation between the project’s expected benefit and the risk for the research participants.