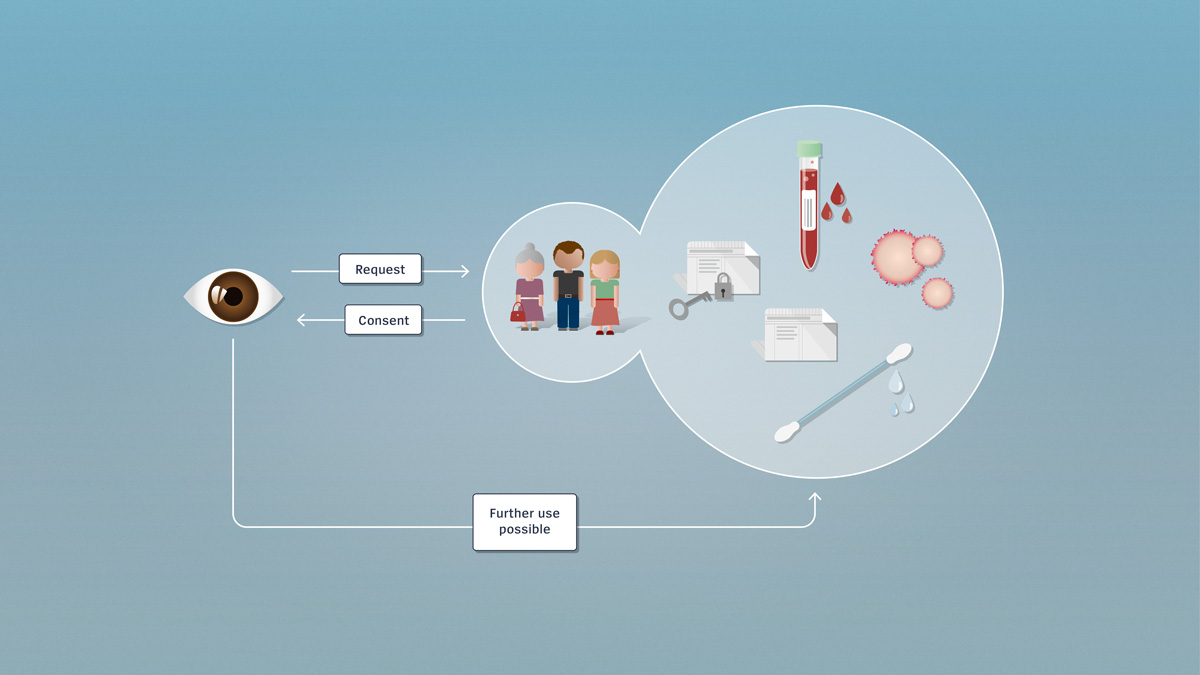
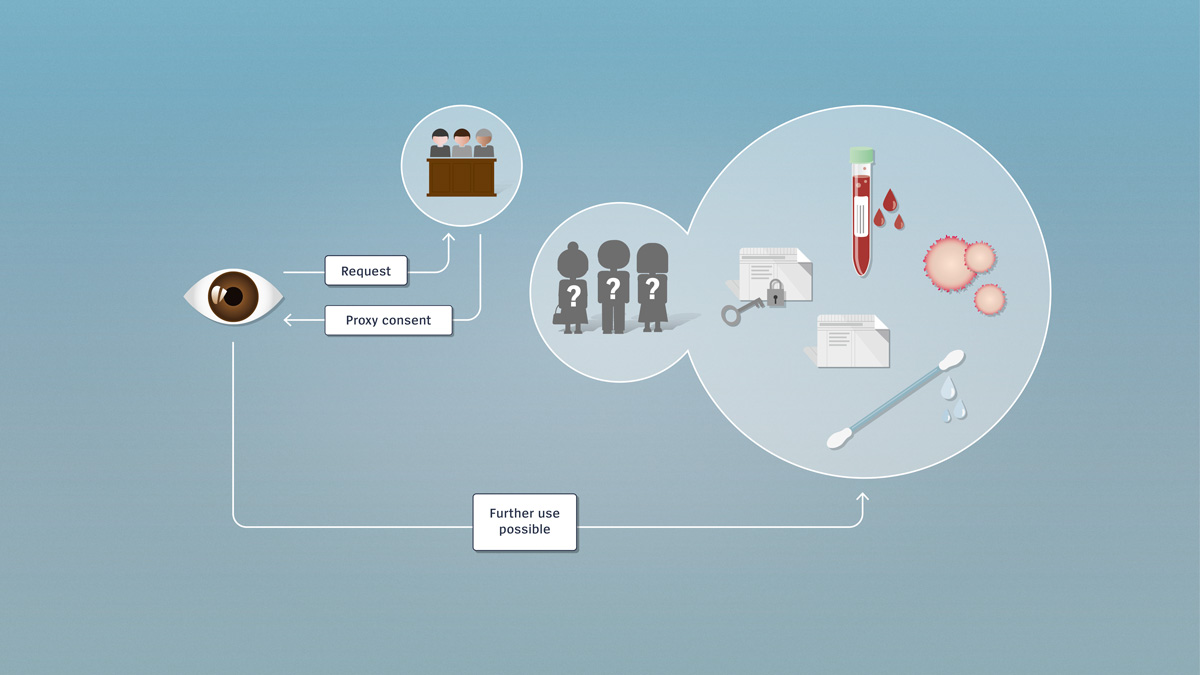
Encrypted and unencrypted data
Research involving further use utilises encrypted or unencrypted health-related data or biological material which, if needed, can be traced back to the donor. Further use of the data for research purposes requires the consent of the person concerned.
Post hoc consent
Since research involving further use utilises pre-existing data or material, the persons involved don’t necessarily know in advance that their material or their data are to be used for research. If no consent has been received, the researcher must contact these persons directly and obtain post hoc consent for further use. Once the researcher has obtained the consent of the persons concerned, he or she may make further use, for research purposes, of the data or biological material.